Assess antigen structures while adsorbed on aluminium hydroxide
Colloidal aluminium salts such as aluminum hydroxide are routinely used to improve antigen immunogenicity. Adsorption affects protein conformation in a range of different ways, and can increase or decrease its stability, depending on its physicochemical properties. Therefore, analyzing the protein in this adsorbed state is crucial, but very challenging due to the scattering properties of the adjuvant, so most of the methods currently used require a desorption step.
We used our expertise in fourier-transform infrared (FTIR) spectroscopy, to develop a methodology to monitor protein stability is its adsorbed state, without desorption.
An example on the thermostability of Bovine Serum Albumin (BSA) is presented here. The first figure below shows FTIR spectra of BSA adsorbed onto aluminium hydroxide as a function of temperature. Spectral variations associated with structural changes are evidenced on this figure.
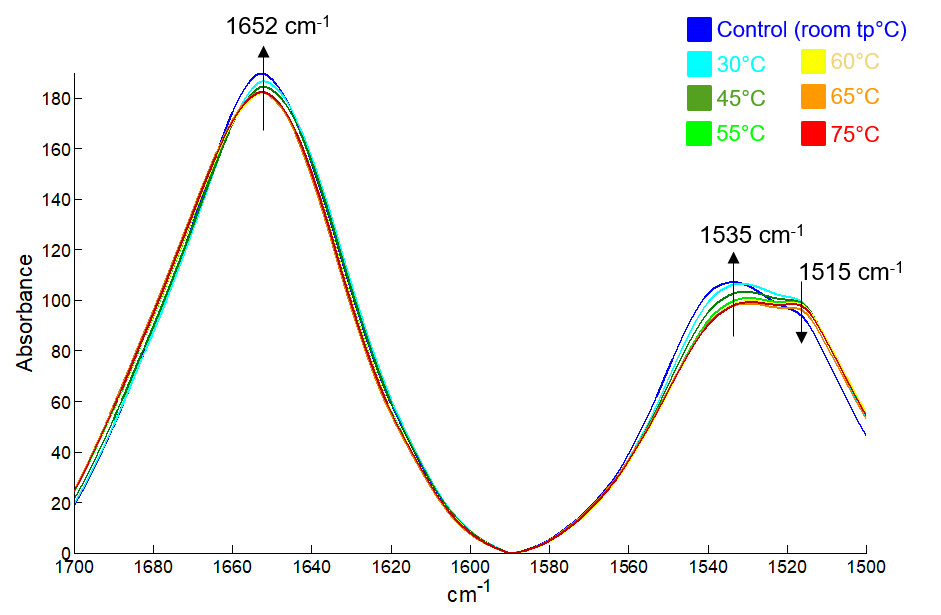
FTIR spectra of progressively heated BSA adsorbed onto aluminium hydroxide gradually coloured from blue to red as indicated in the upper right corner.
Based on these spectral variations, a stability index can be calculated to obtain denaturation curves as presented in the second figure for Human Serum Albumin (not adsorbed) and Bovine Serum Albumin adsorbed onto aluminium hydroxide. Other stresses such as pH, freeze-thaw cycles can also be applied to analyse structural stability with our methodology. Similarly, we can study short- and long-term storage stability of antigens adsorbed onto aluminium-containing adjuvant.
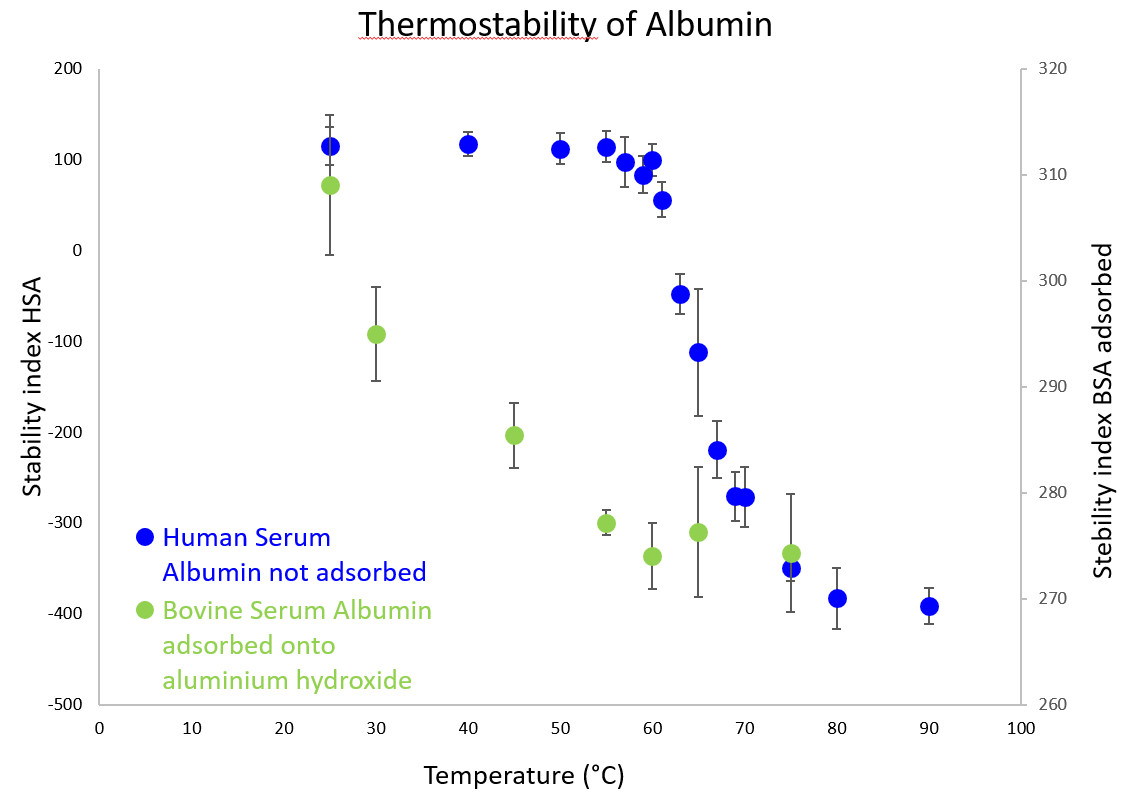
Stability indexes of HSA not adsorbed (blue) and BSA adsorbed onto aluminium hydroxide (green) obtained by FTIR spectroscopy according to temperature. Each point corresponds to the mean of all the spectra recorded at one temperature. Error bars indicate the standard deviation.