Determining batch-to-batch consistency of lipid-to-protein ratio in virus-like particles
In recent years, virus-like particles (VLP) have emerged as a safe and effective advanced vaccine platform. However, the characterization of VLP preparations remains a challenge, due to their large size, diversity and complexity.
Fourier transform infrared (FTIR) spectroscopy can be used to compare the protein structure and the lipid and protein ratio of VLPs and can, therefore, be used as a tool to monitor the quality control of VLP-based vaccines.
The aim is to compare four production batches of a VLP-based vaccine named “VLPB” which was produced with two different processes (i.e. P1 and P2). The vaccine is composed of an enveloped VLP consisting of a lipid membrane in which immunogenic proteins are embedded. The VLP-based vaccines are characterized through the determination of the lipid/protein ratio on the basis of their infrared (IR) spectra. Therefore, the ratio between the peak areas of the lipid and protein absorption bands is calculated for each sample. This ratio is used as a comparative parameter relying on the VLP composition.
It was found that the batches produced with the same process showed similar lipid/protein ratios whereas the change of process induced a significant difference in the lipid/protein ratio.
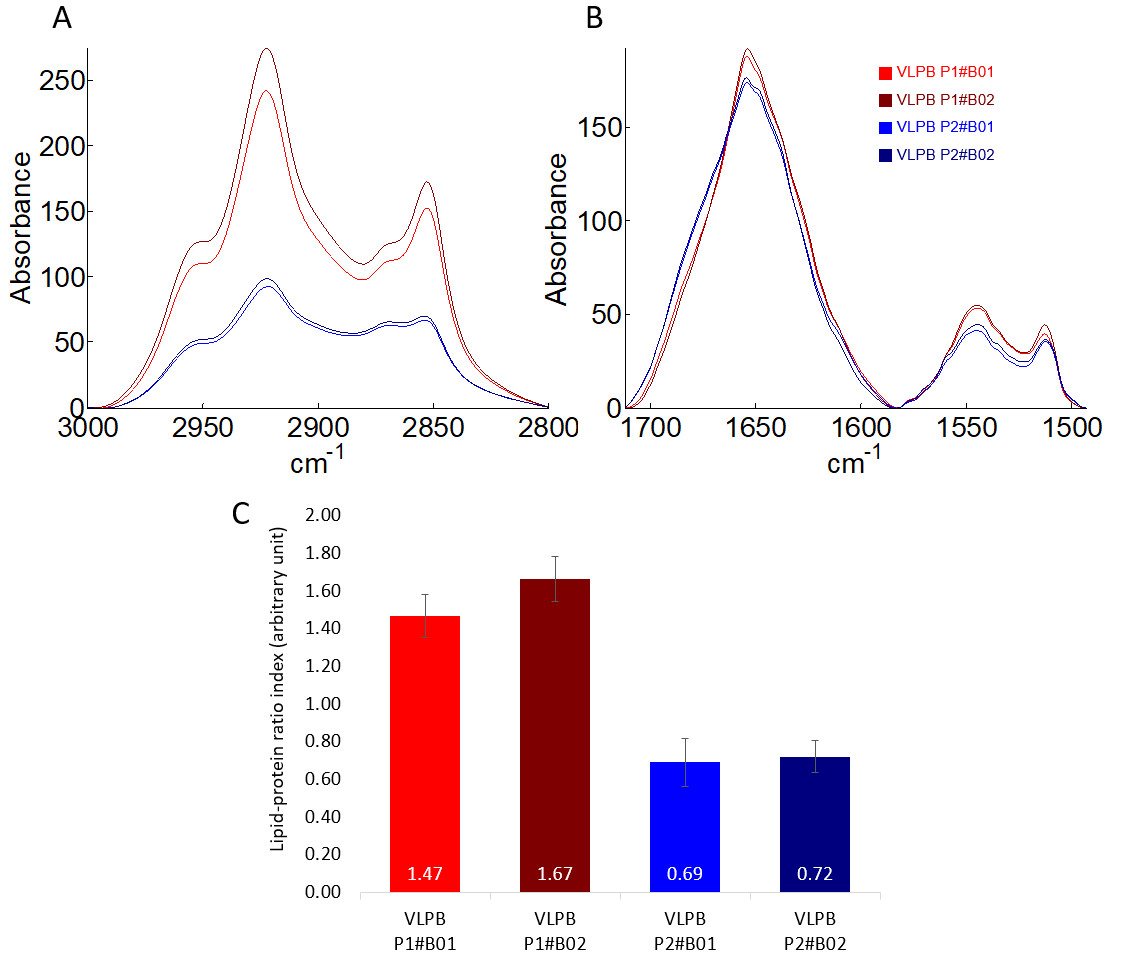
Comparison of the mean spectra (average of 12 spectra per protein and after preprocessing steps) recorded for each batch between 3000-2800 cm-1 (A – spectral region related to lipids absorption) and 1712-1493 cm-1 (B – spectral related to proteins absorption) as well as the mean values of the calculated lipid/protein ratio of all batches with the standard deviations indicated as error bars (C). Each sample is identified by a unique color indicated in the legend.
References
C. L. Effio and J. Hubbuch, “Next generation vaccines and vectors: Designing downstream processes for recombinant protein-based virus-like particles,” Biotechnol. J., vol. 10, pp. 715–727, 2015.
B. C. Buckland, “The process development challenge for a new vaccine.,” Nat. Med., vol. 11, no. 4, pp. S16–S19, 2005.