Fourier-Transform Infrared Spectroscopy
Advantages
Unlike conventional analytical method, FTIR spectroscopy offers unique benefits:
- Robust and unique fingerprint of your sample: sensible to the slightest variation
- Fast analysis: results delivered in a couple of minutes only
- User-friendly and direct technique: no need for extensive sample pre-treatment, physical separation or recurring calibration
- Care for the preservation of your precious samples: 50μg is sufficient
- Multimodal information of critical quality attributes: structural integrity, composition and quantification of post-translational modifications, total protein concentration, quantification of key excipients.
Multipurpose methods
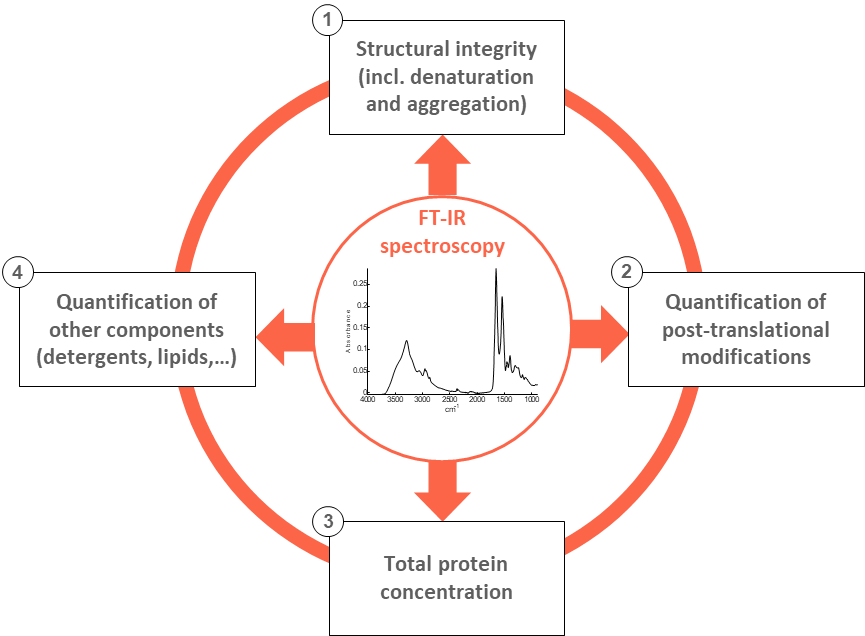
Without any physical separation (chromatographic methods), information related to four critical quality attributes can thus be obtained:
- Higher order structure (absence of denaturation and aggregation);
- Composition and quantification of post-translational modifications (glycosylations, phosphorylations,…);
- Overall protein concentration;
- Quantification of key excipients such as detergents (Tween,…), stabilizing agents or lipids.
Principles
FTIR spectroscopy relies on the fact that most molecules absorb light in the infrared region of the electromagnetic spectrum, converting it to molecular vibrations. In fact, IR light interacts with a chemical bonds only if the energy of the radiation matches the vibrational transition energy of this bond. The absorption wavenumber depends on the exact structure of the molecules.
FTIR spectroscopy has been widely used to characterize biopharmaceuticals for many years, in particular to analyze protein structure.
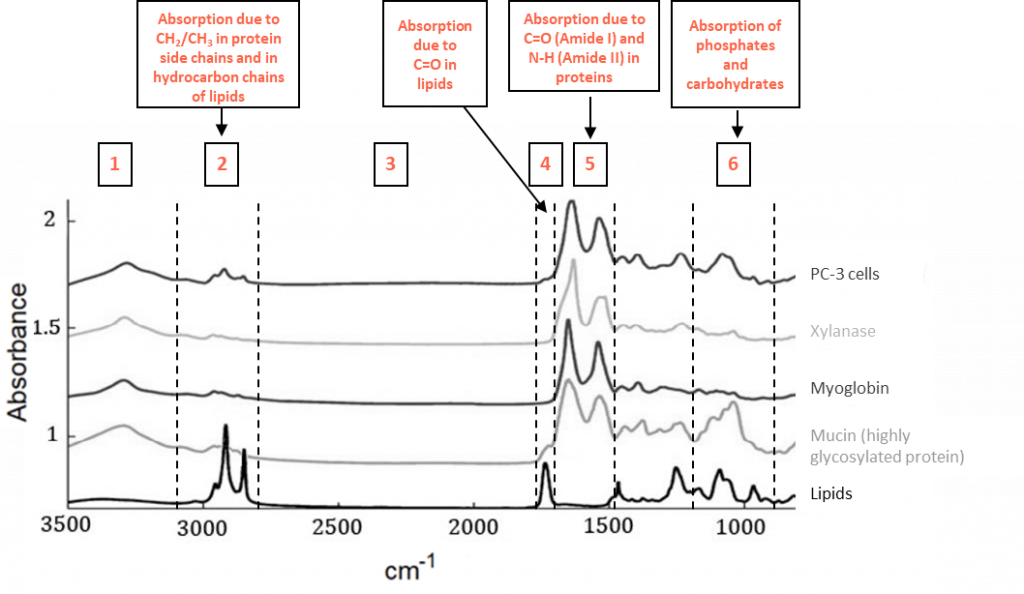
The FTIR spectra of biomolecules can generally be divided in six zones and can generally be assigned as described here below:
- The area between 4000 and 3100 cm-1 is dominated by the absorption arising from –OH and –NH stretching mode. This region is often overlapped by liquid water absorption.
- Between 3100 and 2800 cm-1, the absorptions are mainly due to C-H vibrations of CH2/CH3 present in protein side chains and in hydrocarbon chains of lipids, fatty acids or detergents.
- There is almost no absorption caused by biological molecules between 2800 and 1800 cm-1. In practice, a double peak is observed around 2300 cm-1 which can be assigned to atmospheric CO2 absorption.
- Around 1734 cm-1: ester C=O stretching present in lipids and in some detergents.
- Between 1700 and 1500 cm-1, vibrations of the amide bonds in proteins result in the largest bands in FTIR spectra of proteins. The exact frequencies of Amide I (mostly C=O stretching, 1695-1615 cm-1) and Amide II (mostly N-H bending, 1550-1520 cm-1) are influenced by the strength of hydrogen bonds involving amide C=O and N-H groups as well as the geometry of the polypeptide chain. Each type of secondary structure is associated with specific frequencies at which Amide I and II occur.
- It is established that phosphate and carbohydrates (associated with glycoproteins) are responsible for absorption between 1200 and 950 cm-1.
Through one measurement, an infrared spectrum can provide a global and unique fingerprint of a sample. This fingerprint reports both the chemical composition and the molecular conformation. This sum of information is demonstrated to be very accurate and sensitive to subtle variations but needs to be interpreted in such a way as to maximize the amount of information that can be retrieved.
Furthermore, FTIR spectroscopy allows to analyze biomolecules in a precise, quick and direct manner (a couple of minutes), with limited sample quantity (<50µg) and without need for extensive sample pre-treatment or recurring calibration.
References:
Groß, P.C.; Zeppezauer, M. Infrared spectroscopy for biopharmaceutical protein analysis. J. Pharm. Biomed. Anal. 2010, 53, 29–36
Goormaghtigh, E.; Derenne, A. FTIR Spectroscopy as a Multi-Parameter Analytical Tool. BioPharm Int. 2017, 30.
Barth, A. Infrared spectroscopy of proteins. Biochim Biophys Acta 2007, 1767, 1073–101