Hydrogen-deuterium exchange
The 1H / 2H exchange kinetic provides information about protein conformation, since the exchange rate depends on the stability of the secondary structure and on the accessibility of the peptide bond to the aqueous environment. It is an efficient way to compare the tertiary structure of proteins in several experimental conditions.
We have demonstrated that FTIR spectroscopy can effectively monitor hydrogen-deuterium exchange to compare the protein structure and conformation under two distinct storage conditions.
Samples are exposed to a flow of nitrogen containing 2H2O vapor. Kinetics of 60 minutes were recorded and evolution of the proportion of exchanged amide bonds were computed.
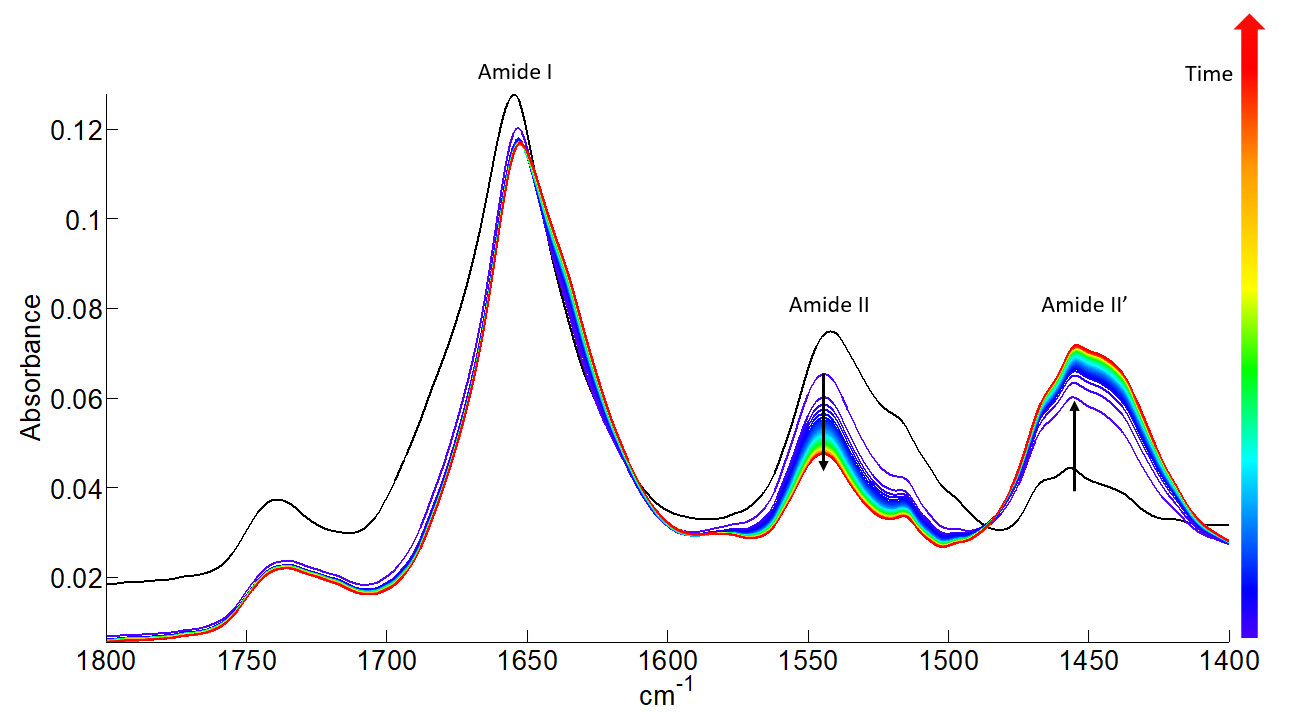
All the FTIR spectra (61) of one kinetics recorded for protein 1 condition 1 sample. The FTIR spectra are gradually coloured from blue to red according to increasing deuteration time.
Upon H-D exchange, the intensity and position of the amide bands are strongly affected. The Amide II band around 1542 cm-1, which is mainly due to the in-plane N-H bending vibration of the peptide bond, shifts toward lower wavenumbers by the H-D exchange to form a new band called Amide II’ (1454 cm-1). It is easily seen that the intensity of the Amide II band decreases while that of the Amide II’ band increases during the deuteration process.
The percentage of amide protons that are exchanged during the deuteration can be determined as a function of time from the ratio of the areas of Amide II and Amide I bands.
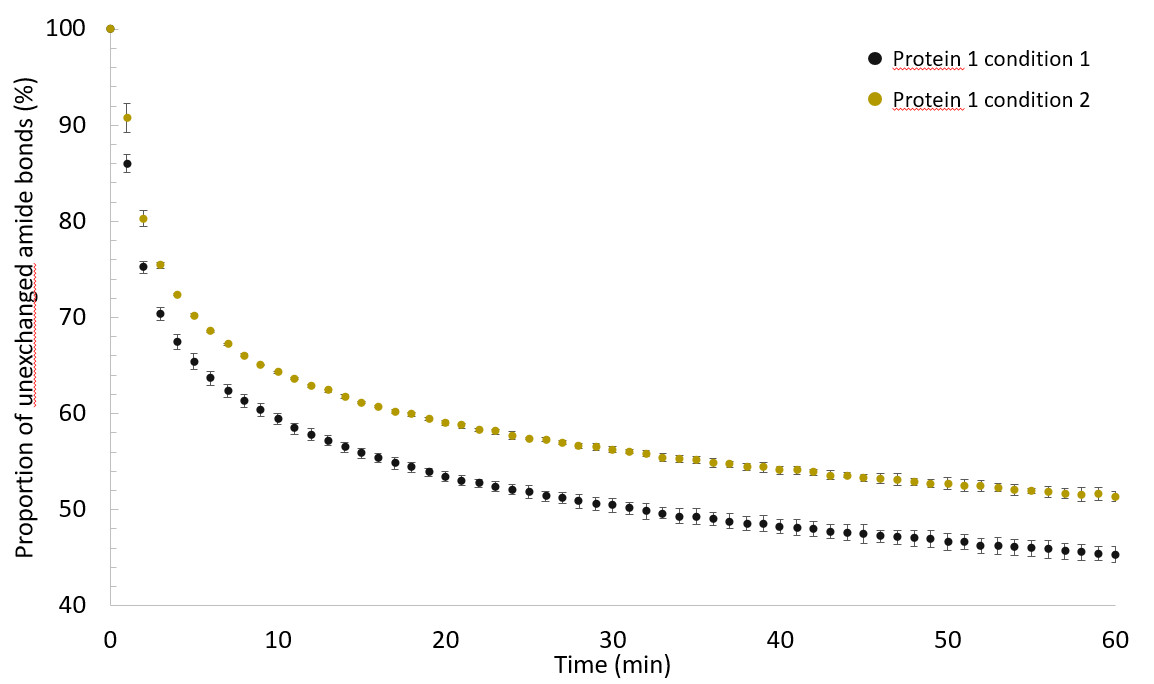
Evolution of the proportion of unexchanged amide bonds computed between 0 and 100% as a function of the deuteration time reported between 0 and 60 minutes for each condition of protein 1. Vertical bars indicate the standard deviation (SD) obtained from three independent experiments (mean ± 1SD).
It was found that 1H/2H exchange kinetics were slower for the protein 1 in condition 2 than in condition 1. We have thus evidence that the storage conditions affect the structure and conformation of protein 1.
References
V. Raussens, J.-M. Ruysschaert, E. Goormaghtigh, Analysis of 1H/2H exchange kinetics using model infrared spectra., Appl. Spectrosc. 58 (2004) 68–82. https://doi.org/10.1366/000370204322729496.
C. Vigano, M. Smeyers, V. Raussens, F. Scheirlinckx, J.M. Ruysschaert, E. Goormaghtigh, Hydrogen-Deuterium Exchange in Membrane Proteins Monitored by IR Spectroscopy: A New Tool to Resolve Protein Structure and Dynamics, in: Biopolymers, 2004: pp. 19–26. https://doi.org/10.1002/bip.20035.